How Water Electrolysis Hydrogen Generators Work: The Complete Guide to Clean Energy Production
Water electrolysis hydrogen generators represent one of the most promising technologies for producing clean, renewable energy. These innovative systems split water molecules into hydrogen and oxygen using electrical energy, creating a sustainable fuel source that’s gaining traction worldwide. As we face growing environmental challenges and the urgent need for carbon-neutral energy solutions, understanding how these generators work becomes increasingly important.

Understanding the Fundamentals of Water Electrolysis
What is Water Electrolysis?
Water electrolysis is an electrochemical process that uses electrical energy to decompose water (H₂O) into its constituent elements: hydrogen (H₂) and oxygen (O₂). This process occurs when an electric current passes through water containing dissolved ions, causing the water molecules to split at the molecular level.
The basic chemical equation for water electrolysis is: 2H₂O + electrical energy → 2H₂ + O₂
This seemingly simple reaction forms the foundation of hydrogen production technology that’s revolutionizing how we think about clean energy storage and distribution.
The Science Behind Molecular Separation
When electrical current flows through water, it creates an oxidation-reduction reaction. At the cathode (negative electrode), water molecules gain electrons and form hydrogen gas. Simultaneously, at the anode (positive electrode), water molecules lose electrons and produce oxygen gas.
The process requires pure water mixed with an electrolyte solution to conduct electricity effectively. Without these dissolved ions, pure water wouldn’t conduct electricity well enough to sustain the electrolysis reaction.
Core Components of Hydrogen Generators
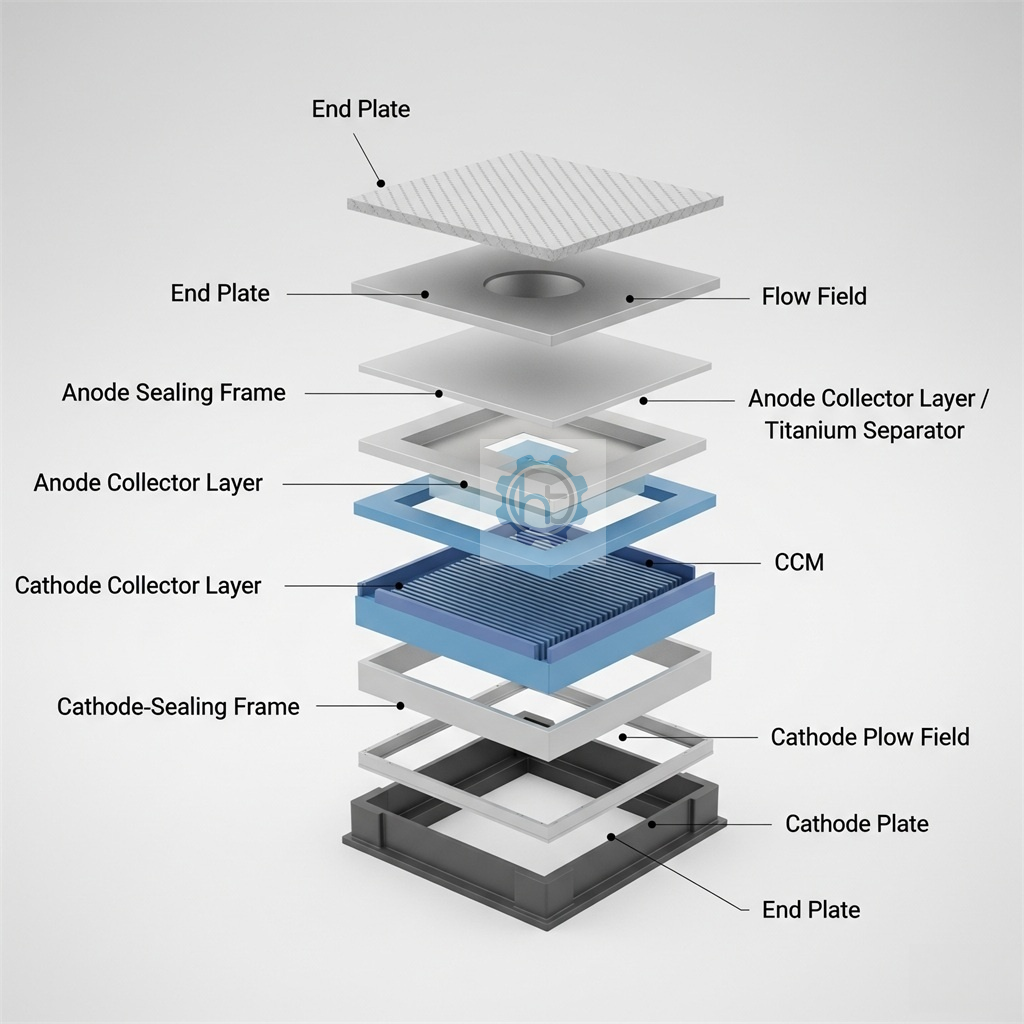
Electrolytic Cells: The Heart of the System
The electrolytic cell contains two electrodes separated by an electrolyte solution. These cells are engineered with specific materials that optimize the electrolysis process while ensuring durability and efficiency.
Modern hydrogen generators typically use multiple cells connected in series or parallel configurations to increase hydrogen production rates and improve overall system efficiency.
Electrode Materials and Their Importance
Cathode Design and Function
The cathode, typically made from materials like nickel, stainless steel, or specialized alloys, serves as the site where hydrogen gas forms. The material choice affects the generator’s efficiency, longevity, and maintenance requirements.
Advanced cathode designs incorporate catalysts that reduce the energy needed for hydrogen production, making the process more economically viable.
Anode Construction and Role
The anode, where oxygen production occurs, must resist corrosion from the oxidizing environment. Materials like titanium with specialized coatings or dimensionally stable anodes (DSA) are commonly used.
The anode design directly impacts the generator’s lifespan and operational costs, as it experiences the most chemically aggressive conditions during electrolysis.
Electrolyte Solutions and Their Properties
Alkaline Electrolytes
Most commercial hydrogen generators use alkaline electrolytes, typically potassium hydroxide (KOH) or sodium hydroxide (NaOH). These solutions provide excellent conductivity while operating at relatively moderate temperatures.
Alkaline electrolysis systems offer proven reliability and cost-effectiveness, making them popular for industrial applications.
Polymer Electrolyte Membranes
Advanced systems utilize polymer electrolyte membrane (PEM) technology, which uses a solid polymer membrane instead of liquid electrolyte. This design offers several advantages including higher purity hydrogen production and more compact system designs.
PEM systems can respond quickly to varying power inputs, making them ideal for integration with renewable energy sources like solar or wind power.
Types of Water Electrolysis Technologies
Alkaline Water Electrolysis
Alkaline electrolysis represents the most mature and widely deployed hydrogen production technology. These systems operate at temperatures between 60-80°C and can achieve efficiencies of 60-70%.
The technology’s main advantages include:
- Lower capital costs compared to other methods
- Proven commercial viability
- Long operational lifespans
- Established supply chains for components
However, alkaline systems typically have slower response times to power fluctuations and require more maintenance due to the liquid electrolyte system.

Proton Exchange Membrane (PEM) Electrolysis
PEM electrolysis uses a solid polymer membrane that allows only protons to pass through while blocking gases. This technology operates at lower temperatures (50-80°C) but higher current densities than alkaline systems.
Key benefits include:
- Higher hydrogen purity (>99.9%)
- Faster response to power changes
- More compact designs
- Lower maintenance requirements
The primary drawback is higher initial costs due to expensive catalyst materials like platinum and iridium.
Solid Oxide Electrolysis Cells (SOEC)
SOEC technology operates at high temperatures (700-900°C) and can achieve the highest theoretical efficiencies. These systems can utilize waste heat from industrial processes, making them highly efficient in appropriate applications.
While still emerging commercially, SOEC systems show promise for large-scale industrial hydrogen production where high-temperature waste heat is available.
The Step-by-Step Electrolysis Process
Initial Water Preparation
The process begins with water treatment to remove impurities that could interfere with electrolysis or damage equipment. Deionized or distilled water is mixed with the appropriate electrolyte to create the conducting solution.
Water quality directly affects system performance and longevity, making proper preparation crucial for efficient operation.
Electrical Energy Application
When electrical current flows through the electrolyte solution, it creates an electric field between the electrodes. The voltage applied must overcome the thermodynamic potential for water splitting (approximately 1.23 volts) plus additional energy to drive the reaction at practical rates.
Most commercial systems operate at voltages between 1.8-2.2 volts per cell, balancing efficiency with practical hydrogen production rates.
Gas Generation and Collection
Hydrogen Production at the Cathode
At the cathode, the following reaction occurs: 2H₂O + 2e⁻ → H₂ + 2OH⁻
The hydrogen gas bubbles form and rise to the surface, where collection systems capture and direct the gas to storage or processing equipment.
Oxygen Production at the Anode
Simultaneously, at the anode: 2OH⁻ → H₂O + ½O₂ + 2e⁻
The oxygen produced is typically vented to the atmosphere unless captured for industrial use or sale as a byproduct.
Gas Separation and Purification
Modern hydrogen generators include separation systems that ensure gases don’t mix, which could create safety hazards. These systems use physical barriers, pressure differentials, and sometimes additional purification steps.
The hydrogen output typically requires minimal purification for most applications, though some uses may require additional processing to remove trace water vapor or oxygen.
Efficiency Factors and Optimization
Energy Efficiency Considerations
The theoretical minimum energy requirement for water electrolysis is 39.4 kWh per kilogram of hydrogen produced. However, real-world systems typically require 50-70 kWh/kg due to various inefficiencies.
Factors affecting efficiency include:
- Operating temperature and pressure
- Electrode materials and design
- Current density
- Electrolyte concentration
- System maintenance and age
Operating Parameter Optimization
Temperature Control
Higher operating temperatures generally increase efficiency by reducing electrical resistance and improving reaction kinetics. However, elevated temperatures also increase energy requirements for heating and may accelerate component degradation.
Most systems optimize temperature based on their specific design and intended application.
Pressure Management
Operating under elevated pressure can improve efficiency and produce compressed hydrogen directly, reducing downstream compression costs. However, higher pressure systems require more robust and expensive equipment.
The optimal pressure depends on the intended hydrogen use and storage requirements.
Maintenance and Longevity Factors
Regular maintenance ensures optimal performance and extends system life. Key maintenance activities include:
- Electrolyte monitoring and replacement
- Electrode inspection and cleaning
- System leak detection and repair
- Performance monitoring and optimization
Proper maintenance can extend system life to 20-30 years or more, making hydrogen generators a viable long-term investment.
Applications and Uses of Generated Hydrogen
Industrial Applications
Hydrogen produced through electrolysis serves numerous industrial purposes:
Chemical Processing: Hydrogen is essential for ammonia production, petroleum refining, and various chemical synthesis processes.
Metal Processing: The steel industry uses hydrogen for direct reduction of iron ore, offering a cleaner alternative to carbon-based reduction methods.
Electronics Manufacturing: High-purity hydrogen is crucial for semiconductor fabrication and other precision manufacturing processes.
Energy Storage and Grid Balancing
Electrolytic hydrogen generators enable large-scale energy storage by converting excess renewable electricity into hydrogen when production exceeds demand. This stored hydrogen can later generate electricity through fuel cells or combustion turbines.
This application is becoming increasingly important as renewable energy deployment grows and grid operators need flexible storage solutions.
Transportation Fuel
Hydrogen fuel cells power vehicles ranging from cars and buses to trains and ships. Electrolytic hydrogen provides a carbon-neutral fuel source when powered by renewable electricity.
The transportation sector represents a rapidly growing market for clean hydrogen, driving demand for efficient production technologies.
Residential and Commercial Applications
Smaller-scale hydrogen generators serve residential and commercial users for:
- Backup power systems
- Combined heat and power applications
- Off-grid energy solutions
- Industrial process heating
These applications often prioritize reliability and ease of operation over maximum efficiency.
Environmental Impact and Sustainability
Carbon Footprint Considerations
The environmental impact of hydrogen production depends entirely on the electricity source. When powered by renewable energy, electrolytic hydrogen produces zero direct carbon emissions.
However, hydrogen produced using grid electricity may have a significant carbon footprint if the electrical grid relies heavily on fossil fuels.
Life Cycle Assessment
Comprehensive environmental analysis considers the entire system lifecycle, including:
- Manufacturing and installation impacts
- Operational energy consumption
- Maintenance and replacement requirements
- End-of-life disposal and recycling
Well-designed systems powered by renewable energy typically show favorable environmental profiles compared to fossil fuel alternatives.
Water Resource Requirements
Water electrolysis requires approximately 9 liters of pure water per kilogram of hydrogen produced. While this seems substantial, it’s relatively modest compared to other industrial processes.
Most systems can utilize recycled or treated water, minimizing impact on freshwater resources.
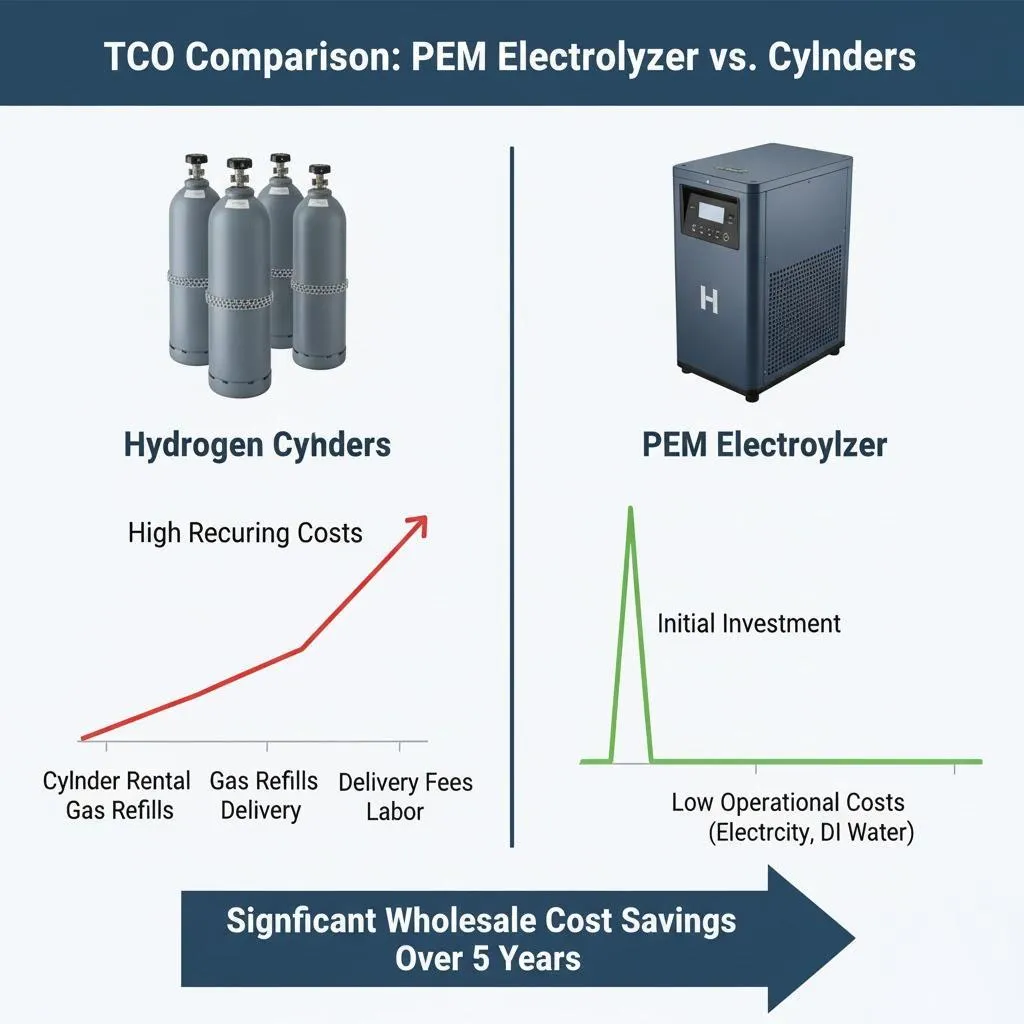
Economic Considerations and Cost Analysis
Capital Investment Requirements
Initial costs for hydrogen generators vary widely based on:
- System size and capacity
- Technology type (alkaline vs. PEM vs. SOEC)
- Installation complexity
- Local labor and material costs
Small residential systems might cost $10,000-50,000, while industrial installations can require millions of dollars in investment.
Operating and Maintenance Costs
Ongoing costs include:
- Electricity consumption (typically 60-80% of total costs)
- Maintenance and replacement parts
- Labor for operation and maintenance
- Insurance and regulatory compliance
Electricity costs dominate the economic equation, making access to low-cost renewable energy crucial for competitive hydrogen production.
Economic Competitiveness
Hydrogen production costs continue declining as technology improves and renewable electricity becomes cheaper. Current costs range from $3-8 per kilogram, with projections suggesting costs below $2/kg by 2030 in optimal locations.
The economic viability depends heavily on local electricity prices, system utilization rates, and available incentives or carbon pricing mechanisms.
Safety Considerations and Risk Management
Hydrogen Safety Properties
Hydrogen presents unique safety considerations:
- Highly flammable with a wide combustion range
- Odorless and colorless, making leaks difficult to detect
- Small molecule size allows permeation through many materials
- Burns with an invisible flame in daylight
Understanding these properties is essential for safe system design and operation.
Safety System Design
Modern hydrogen generators incorporate multiple safety features:
Gas Detection Systems: Sensitive hydrogen detectors monitor for leaks and trigger alarms or automatic shutdowns.
Ventilation Systems: Proper ventilation prevents hydrogen accumulation in enclosed spaces.
Emergency Shutdown Systems: Automated systems can quickly stop hydrogen production and isolate gas supplies during emergencies.
Pressure Relief Systems: Safety valves and rupture discs protect against overpressure conditions.
Regulatory Compliance
Hydrogen generators must comply with various safety codes and standards, including:
- NFPA codes for hydrogen systems
- ASME pressure vessel standards
- Local building and fire codes
- Occupational safety requirements
Compliance ensures safe operation and may be required for insurance coverage and financing.
Future Developments and Innovations
Emerging Technologies
Research continues into advanced electrolysis technologies:
Advanced Electrode Materials: New catalysts and electrode designs promise higher efficiency and longer lifespans.
High-Temperature Systems: Next-generation SOEC systems aim for even higher efficiencies through improved materials and designs.
Integrated Systems: Combining electrolysis with renewable energy generation and energy storage in optimized packages.
Market Trends and Projections
The hydrogen electrolyzer market is experiencing rapid growth, driven by:
- Declining renewable energy costs
- Government policies supporting clean energy
- Corporate commitments to carbon neutrality
- Technological improvements reducing costs
Industry projections suggest dramatic capacity growth over the next decade, with corresponding cost reductions and performance improvements.
Integration with Renewable Energy
Future systems will increasingly integrate with:
- Solar photovoltaic arrays
- Wind turbines
- Grid-scale battery storage
- Smart grid management systems
This integration enables optimal utilization of variable renewable energy sources while providing grid stability services.
Frequently Asked Questions
How efficient are water electrolysis hydrogen generators?
Modern water electrolysis systems typically achieve 60-80% electrical efficiency, meaning they convert 60-80% of the input electrical energy into chemical energy stored in hydrogen. The best commercial systems can reach efficiencies above 80%, while laboratory demonstrations have achieved even higher efficiencies. However, the overall efficiency depends on many factors including operating conditions, system design, and maintenance status.
What is the lifespan of a hydrogen generator?
Well-maintained hydrogen generators typically last 20-30 years, though some components may require replacement more frequently. The electrodes and electrolyte systems usually need maintenance or replacement every 5-10 years, depending on usage intensity and operating conditions. Regular maintenance and proper operation significantly extend system life and maintain performance.
How much does it cost to produce hydrogen through electrolysis?
Current hydrogen production costs through electrolysis range from $3-8 per kilogram, with electricity costs representing 60-80% of the total. Costs vary significantly based on electricity prices, system size, capacity utilization, and technology type. Projections suggest costs could fall below $2/kg by 2030 as renewable electricity becomes cheaper and technology improves.
Can hydrogen generators run on renewable energy?
Yes, hydrogen generators work excellently with renewable energy sources like solar and wind power. In fact, this combination creates truly carbon-neutral hydrogen production. Advanced PEM systems can respond quickly to variable renewable energy output, making them particularly suitable for renewable integration. Many large-scale projects now combine renewable energy directly with hydrogen production.
What are the main safety concerns with hydrogen generators?
The primary safety concerns include hydrogen’s flammability, its tendency to leak through small openings, and its odorless, colorless nature making detection challenging. Modern systems address these concerns through comprehensive safety systems including gas detectors, automatic ventilation, emergency shutdowns, and pressure relief systems. When properly designed and maintained, hydrogen generators have excellent safety records.
How pure is the hydrogen produced by electrolysis?
Electrolysis produces very high-purity hydrogen, typically 99.5-99.9% pure. PEM systems can achieve even higher purities exceeding 99.9%. The main impurities are usually trace amounts of water vapor and oxygen, which can be further reduced through additional purification if needed for specific applications. This high purity makes electrolytic hydrogen suitable for most industrial and fuel cell applications without additional processing.
Conclusion
Water electrolysis hydrogen generators represent a crucial technology for our transition to a clean energy future. These systems offer a reliable, scalable method for producing carbon-neutral hydrogen when powered by renewable electricity. While current costs remain higher than fossil fuel alternatives, rapid technological improvements and declining renewable energy prices are making electrolytic hydrogen increasingly competitive.
The technology’s versatility enables applications ranging from industrial processes and energy storage to transportation fuels and residential power systems. As governments worldwide implement policies supporting clean energy and carbon reduction, hydrogen generators will play an increasingly important role in achieving sustainability goals.
Understanding how these systems work empowers individuals, businesses, and policymakers to make informed decisions about hydrogen technology adoption. Whether considering a small residential system or planning large-scale industrial implementation, the fundamental principles of water electrolysis remain the same: using clean electricity to split water molecules and create the clean fuel of the future.
The future of hydrogen generation looks bright, with ongoing research promising even more efficient, cost-effective, and versatile systems. As we continue developing renewable energy infrastructure and working toward carbon neutrality, water electrolysis hydrogen generators will undoubtedly remain at the forefront of clean energy technology.