As the world grapples with the pressing need for sustainable energy solutions, hydrogen emerges as a promising alternative fuel. Among the various methods of hydrogen production, Proton Exchange Membrane (PEM) electrolysis stands out for its efficiency and environmental benefits. This article delves into the intricacies of PEM electrolysis for hydrogen production, exploring its principles, advantages, and future prospects.
Understanding PEM Electrolysis
PEM electrolysis is a process that utilizes a proton exchange membrane to split water into hydrogen and oxygen. This method is celebrated for its ability to produce high-purity hydrogen efficiently and cleanly. The core components of a PEM electrolyzer include the membrane, electrodes, and catalyst layers, each playing a crucial role in optimizing the electrochemical reactions.
How PEM Electrolysis Works
In a PEM electrolyzer, water is introduced at the anode, where it is oxidized to produce oxygen, protons, and electrons. The protons migrate through the proton exchange membrane to the cathode, where they combine with electrons to form hydrogen gas. This process is facilitated by catalyst layers that enhance reaction rates without consuming additional energy.
Advantages of PEM Electrolysis
PEM electrolysis offers several advantages over traditional hydrogen production methods like alkaline electrolysis and steam methane reforming (SMR). Here are some key benefits:
- High Purity Hydrogen: PEM electrolysis produces hydrogen with high purity, reducing the need for downstream purification and saving energy and costs.
- Efficient and Scalable: The process is highly efficient, thanks to advanced materials and design. It is also scalable, making it suitable for both small-scale and industrial applications.
- Environmental Benefits: Unlike SMR, which emits significant carbon dioxide, PEM electrolysis emits only oxygen as a by-product, making it a cleaner option for hydrogen production.
- Fast Response Times: PEM systems can quickly adjust to changes in energy input, making them ideal for integration with renewable energy sources like wind and solar.
Key Materials in PEM Electrolysis
The efficiency and durability of PEM electrolysis heavily depend on the materials used. The proton exchange membrane, typically made from Nafion, is renowned for its strong proton conductivity and chemical stability. Catalysts, often platinum or iridium, are used to accelerate electrochemical reactions, although research is underway to find cost-effective alternatives.
Innovations in Material Science
Recent advancements in material science have led to the development of non-precious metal alloys and nanostructures that offer similar efficiency at a lower cost. Improved membrane technology has also enhanced proton conductivity and stability under various operational conditions.
Proven Strategies for Cost Reduction
To make PEM electrolysis more economically viable, several cost-reduction strategies have been implemented:
- Material Optimization: Using high-quality materials enhances efficiency and longevity, lowering operational costs.
- Economies of Scale: As production volumes increase, the cost per unit decreases, making the technology more affordable.
- Efficiency Improvements: Advanced maintenance practices and the use of renewable energy sources help reduce energy costs.
- Research and Development: Ongoing R&D efforts are crucial for discovering new materials and processes that enhance performance and reduce costs.
The Role of PEM Electrolysis in Sustainability
PEM electrolysis plays a pivotal role in reducing carbon emissions from energy production. By utilizing renewable energy sources like wind and solar, PEM electrolyzers generate hydrogen without CO2 emissions, contributing to a cleaner and more sustainable energy landscape.
Future Prospects
The future of PEM electrolysis looks promising, with continuous advancements in materials and technology driving efficiency and cost reductions. As renewable energy becomes more prevalent, PEM hydrogen generators could provide reliable and eco-friendly energy storage and supply, supporting the global transition to sustainable energy systems.
Challenges and Considerations
Despite its advantages, PEM electrolysis faces challenges that need to be addressed to enhance its commercial viability:
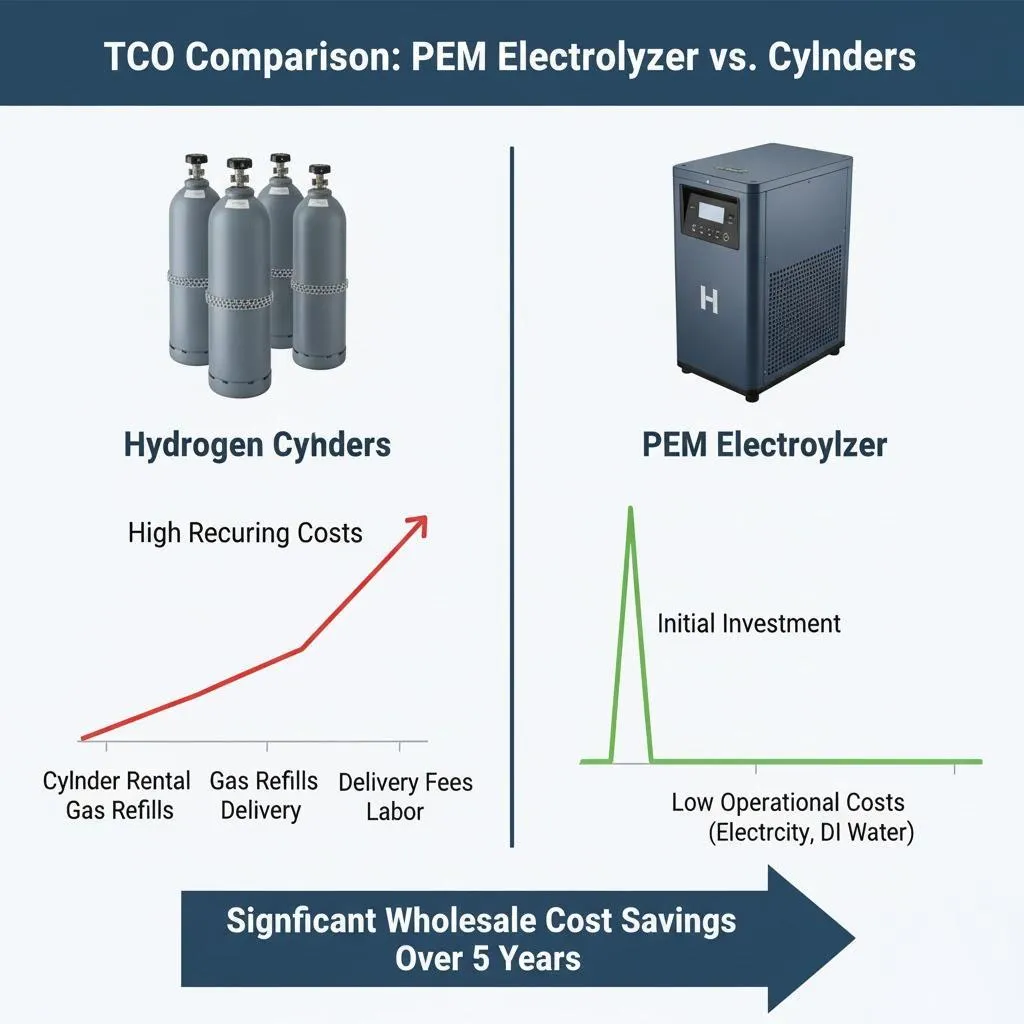
- High Initial Costs: The use of precious metals and advanced materials increases the initial investment required for PEM systems.
- Durability Concerns: The harsh operating conditions can lead to the degradation of components, necessitating ongoing research to improve durability.
- Scalability: While PEM systems are scalable, achieving widespread adoption requires overcoming technical and economic barriers.
Conclusion
PEM electrolysis for hydrogen production represents a significant step towards a sustainable energy future. Its ability to produce high-purity hydrogen efficiently and cleanly makes it an attractive option for various applications, from industrial processes to renewable energy integration. As research and innovation continue to advance this technology, PEM electrolysis is poised to play a crucial role in the global shift towards cleaner energy sources.
For those interested in exploring the potential of PEM electrolysis further, consider delving into the latest research and developments in this exciting field. By staying informed and engaged, we can collectively contribute to a more sustainable and energy-efficient world.
Call to Action
If you found this article informative, please share it with others who may be interested in sustainable energy solutions. For more insights and updates on hydrogen production and renewable energy technologies, subscribe to our newsletter or follow us on social media.