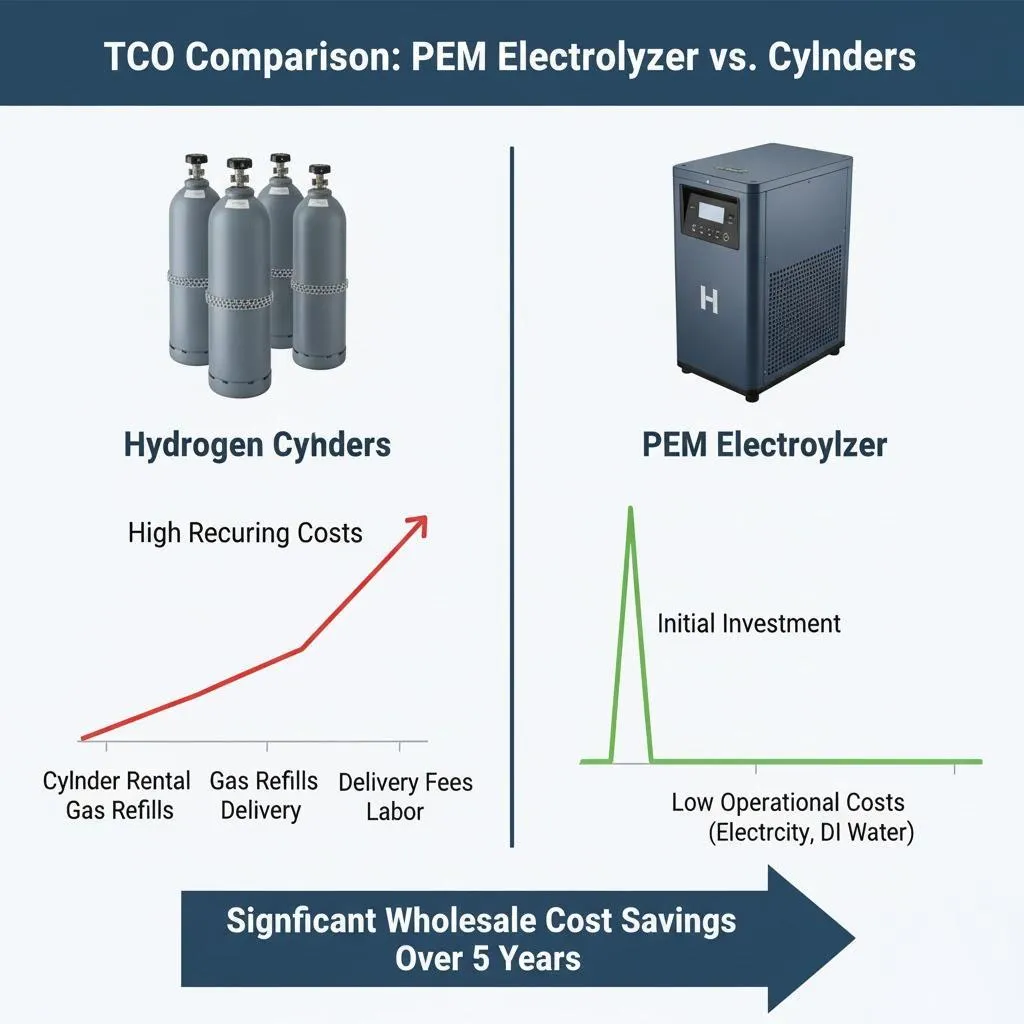
Introduction
In the quest for sustainable energy solutions, hydrogen has emerged as a promising candidate. Among the various methods of hydrogen production, alkaline electrolysis stands out due to its efficiency and historical significance. First observed in 1800, this method separates oxygen and hydrogen from water using an electric current in an alkaline solution. This article delves into the intricacies of alkaline electrolysis, exploring its principles, advantages, challenges, and future prospects.
What is Alkaline Electrolysis?
Alkaline electrolysis is a process that uses an alkaline electrolyte, typically potassium hydroxide (KOH) or sodium hydroxide (NaOH), to conduct electricity between two electrodes submerged in water. When an electric current is applied, water molecules are split into hydrogen and oxygen gases. This method is characterized by its use of non-noble metal catalysts, making it a cost-effective solution for hydrogen production.
Key Components of Alkaline Electrolysis
- Electrodes: Typically made from nickel-based metals, these are essential for conducting electricity and facilitating the electrochemical reactions.
- Diaphragm: A non-conductive, porous separator that prevents electrical shorts and gas mixing while allowing hydroxide ions to pass through.
- Alkaline Electrolyte: A solution of KOH or NaOH, which facilitates ionic conductivity and supports the electrochemical reactions.
The Science Behind Alkaline Electrolysis
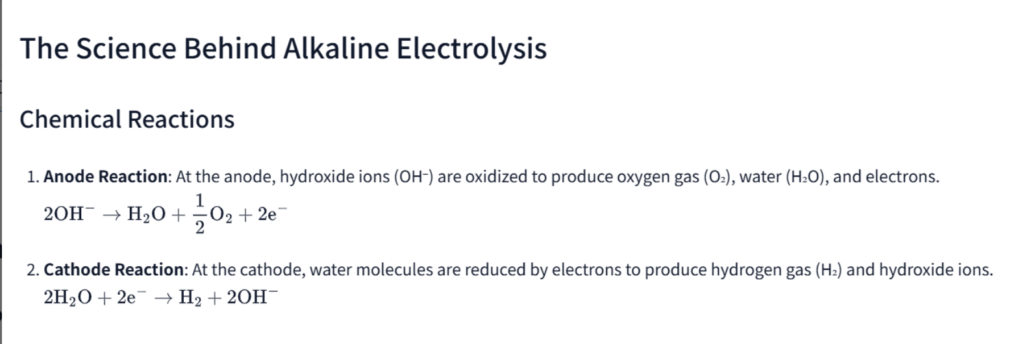
Chemical Reactions
- Anode Reaction: At the anode, hydroxide ions (OH⁻) are oxidized to produce oxygen gas (O₂), water (H₂O), and electrons. [ 2 \text{OH}^- \rightarrow \text{H}_2\text{O} + \frac{1}{2}\text{O}_2 + 2\text{e}^- ]
- Cathode Reaction: At the cathode, water molecules are reduced by electrons to produce hydrogen gas (H₂) and hydroxide ions. [ 2 \text{H}_2\text{O} + 2\text{e}^- \rightarrow \text{H}_2 + 2 \text{OH}^- ]
Efficiency and Performance
State-of-the-art alkaline electrolysis systems can achieve cell voltage efficiencies of 52-69% and specific energy consumption rates ranging from 4.5 to 7.0 kWh/Nm³. These systems operate at temperatures between 60-80°C and pressures below 30 bar, with a lifetime of up to 90,000 hours for the stack.
Historical Context and Evolution
Early Developments
Alkaline electrolysis was first demonstrated by William Nicholson and Sir Anthony Carlisle in 1800. This pioneering work laid the groundwork for subsequent advancements in electrochemical hydrogen production.
Technological Milestones
- 1833: Michael Faraday’s discovery of the Faraday constant and electrochemical nomenclature.
- 1889: Hermann Nernst’s formulation of the Nernst equation, crucial for understanding electrochemical potentials.
- 1955: The first large-scale pressurized electrolysis installation in Peru, marking a significant industrial application.
Recent Innovations
Recent efforts have focused on enhancing the compatibility of alkaline electrolysis with intermittent energy sources. One promising approach is magnetic activation, which targets heating within the electrolyzer to improve efficiency and adaptability.
Advantages of Alkaline Electrolysis
Cost-Effectiveness
Compared to polymer electrolyte membrane (PEM) electrolysis, alkaline electrolysis uses cheaper catalysts, reducing overall production costs.
Durability and Gas Purity
The use of an exchangeable electrolyte and stable nickel-based catalysts enhances durability. Additionally, the lower gas diffusivity in alkaline electrolytes results in higher gas purity.
Scalability
Alkaline electrolysis systems are easily scalable, making them suitable for both small-scale and industrial applications.
Challenges and Limitations
Intermittency Issues
One major challenge is the system’s inertia, which limits its ability to pair with intermittent renewable energy sources. The low mobility of hydroxide ions and high concentrations used necessitate a stationary system for optimal performance.
Material and Efficiency Constraints
While nickel-based catalysts are cost-effective, they are less active than noble metals, potentially limiting efficiency. Research into alternative materials and catalyst structures is ongoing to address these limitations.
Future Prospects and Innovations
Integration with Renewable Energy
Advancements in pressure control and magnetic activation are paving the way for more efficient integration with renewable energy sources. Dome-loaded back pressure controllers, for instance, play a crucial role in maintaining optimal operating conditions.
Industrial Applications
As industries increasingly seek sustainable hydrogen production methods, alkaline electrolysis is poised to play a central role. Its applications range from renewable energy storage to fuel cell technologies and industrial hydrogen supply.
Conclusion
Alkaline electrolysis remains a cornerstone of hydrogen production, offering a sustainable and efficient method for generating this clean energy source. While challenges persist, ongoing research and technological innovations continue to enhance its viability and integration with renewable energy systems. As the demand for clean energy solutions grows, alkaline electrolysis will undoubtedly play a pivotal role in the transition to a more sustainable energy future.