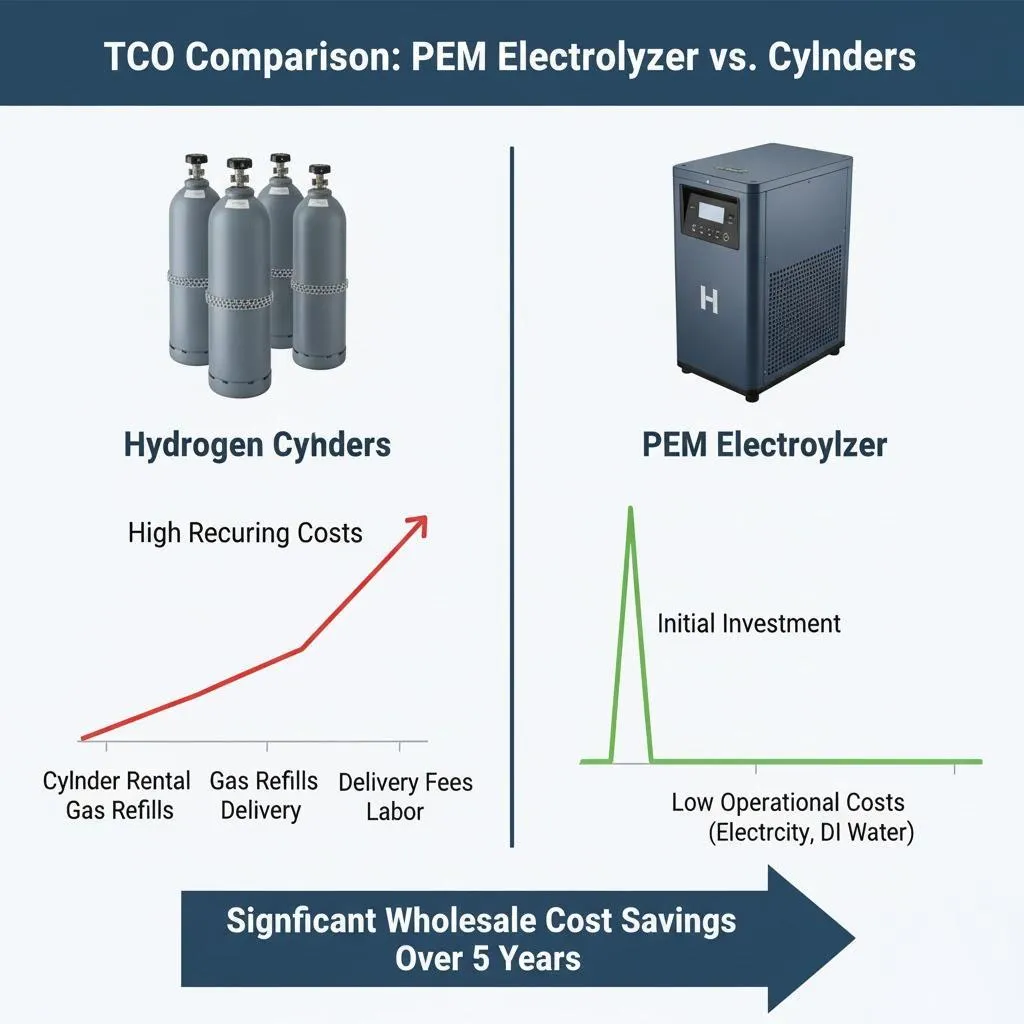
In the quest for sustainable and clean energy solutions, hydrogen production through PEM electrolysis stands out as a promising technology. Proton Exchange Membrane (PEM) electrolysis is an advanced method of splitting water into hydrogen and oxygen using a solid polymer membrane. This process not only offers high efficiency but also aligns with global efforts to reduce carbon emissions. In this article, we delve into the intricacies of PEM electrolysis, exploring its principles, benefits, and potential impact on the energy landscape.
What is PEM Electrolysis?
PEM electrolysis is a cutting-edge technology that uses a proton exchange membrane to facilitate the electrolysis of water. At the core of this process is a solid polymer membrane that conducts protons while blocking electrons, efficiently separating hydrogen and oxygen gases. The process involves two half-reactions: the Oxygen Evolution Reaction (OER) at the anode and the Hydrogen Evolution Reaction (HER) at the cathode. Water is oxidized at the anode to produce oxygen, protons, and electrons. These protons move through the membrane to the cathode, where they combine with electrons to form hydrogen gas.
Key Components and Materials
The efficiency and durability of PEM electrolysis largely depend on the materials used in its construction. The proton exchange membrane is typically made from perfluorosulfonic acid polymers like Nafion, known for their excellent proton conductivity and chemical stability. The catalysts used in PEM electrolyzers are often precious metals like platinum and iridium, which play a crucial role in enhancing the electrochemical reactions.
- Anode Catalyst: Iridium-based catalysts are commonly used for the OER due to their high activity and stability.
- Cathode Catalyst: Platinum is the preferred choice for the HER, offering high purity and efficiency in hydrogen production.
- Membrane: Nafion is the most widely used membrane, providing a balance of durability and performance.
Advantages of PEM Electrolysis
PEM electrolysis offers several advantages over traditional hydrogen production methods, such as alkaline electrolysis and steam methane reforming:
- High Efficiency: PEM electrolysis boasts higher efficiency, converting more electricity into hydrogen.
- Compact Design: The technology’s compact size allows for easier integration into various systems, enhancing its versatility.
- Fast Response Time: PEM electrolyzers can quickly adapt to changes in power input, making them ideal for use with renewable energy sources like wind and solar.
- Environmental Benefits: This method produces hydrogen without greenhouse gas emissions, contributing to a cleaner environment.
- High Purity Hydrogen: The solid polymer membrane ensures minimal gas crossover, resulting in high purity hydrogen output.
Challenges and Considerations
Despite its advantages, PEM electrolysis faces certain challenges that need to be addressed for wider adoption:
- Cost: The use of precious metals like platinum and iridium increases the cost of PEM electrolyzers. Efforts are underway to develop alternative catalysts to reduce expenses.
- Durability: While PEM electrolyzers are efficient, their lifespan is generally shorter than that of alkaline electrolyzers. Research is focused on enhancing the durability of key components.
Environmental Impact and Sustainability
PEM electrolysis is a game-changer in the transition to sustainable energy. By utilizing renewable electricity sources, such as wind or solar, PEM electrolyzers can produce green hydrogen, significantly reducing carbon emissions. This technology not only supports the global shift towards low-carbon solutions but also offers a pathway to achieving energy independence.
Future Prospects and Market Trends
The future of PEM electrolysis looks promising, with increasing investments and advancements in material science. As the demand for clean energy solutions grows, PEM technology is expected to play a pivotal role in hydrogen production. Companies like Dakenchem and Johnson Matthey are at the forefront of developing innovative materials that enhance the performance and cost-effectiveness of PEM electrolyzers.
Conclusion
PEM electrolysis represents a significant leap forward in the production of clean hydrogen. With its high efficiency, environmental benefits, and potential for integration with renewable energy sources, PEM electrolysis is poised to become a cornerstone of the sustainable energy landscape. As research and development continue to address current challenges, the widespread adoption of this technology could revolutionize the way we produce and utilize hydrogen.
In conclusion, embracing PEM electrolysis not only aligns with global sustainability goals but also offers a viable solution to the growing demand for clean and efficient energy. For those interested in exploring the potential of PEM electrolysis further, consider reaching out to industry experts or delving into additional resources on this transformative technology.