1. Introduction to PEM Electrolyzers
Proton Exchange Membrane (PEM) electrolysis is a cutting-edge technology for producing high-purity hydrogen from water and electricity. At its core, PEM electrolysis utilizes a specialized solid polymer membrane that selectively allows protons to pass through while separating the produced hydrogen and oxygen gases.
The importance of PEM electrolyzers in the burgeoning field of green hydrogen production cannot be overstated. As industries worldwide seek to decarbonize and transition towards sustainable energy sources, PEM technology offers a highly efficient and flexible method to generate hydrogen using renewable electricity (e.g., solar, wind). This “green” hydrogen is a crucial enabler for a net-zero future.
This guide is specifically tailored for industrial buyers and decision-makers in sectors such as renewable energy, chemical manufacturing, transportation, and power generation. We aim to provide a clear understanding of how PEM electrolyzers operate, their benefits, and key considerations for investment.

2. Key Components of a PEM Electrolyzer
A PEM electrolyzer stack is comprised of several critical components working in concert:
- Proton Exchange Membrane (PEM): This is the heart of the electrolyzer. It’s a specially treated, solid polymer material (often a Nafion™-based material) that is impermeable to gases but an excellent conductor of protons (H⁺ ions) when hydrated. It also acts as the electrolyte, eliminating the need for liquid electrolytes like those in alkaline systems.
- Electrodes (Anode & Cathode):
- Anode (Oxygen Electrode): This is where water oxidation occurs, producing oxygen, protons, and electrons. It typically uses catalysts based on precious metals like iridium to facilitate the oxygen evolution reaction (OER).
- Cathode (Hydrogen Electrode): This is where protons and electrons combine to form hydrogen gas. Platinum-based catalysts are commonly used here for the hydrogen evolution reaction (HER).
- Anode (Oxygen Electrode): This is where water oxidation occurs, producing oxygen, protons, and electrons. It typically uses catalysts based on precious metals like iridium to facilitate the oxygen evolution reaction (OER).
- Bipolar Plates: These plates, typically made of titanium or stainless steel, perform multiple functions. They provide structural support, distribute reactants (water) and current evenly across the cell area, and separate individual cells in a stack while electrically connecting them in series. They also have flow channels machined into them for water supply and gas removal.
- Gas Diffusion Layers (GDLs): Located between the electrodes and bipolar plates, GDLs are porous materials (often carbon paper or cloth for the cathode, and sintered titanium for the anode) that facilitate the uniform distribution of water to the catalyst sites and the efficient removal of product gases (H₂ and O₂). They also provide electrical contact between the electrodes and bipolar plates.
- Current Collectors: Usually part of the bipolar plate assembly or end plates, these components ensure efficient distribution of electrical current to the electrodes.
3. How PEM Electrolysis Works: Step-by-Step Process
The magic of PEM electrolysis lies in its sophisticated yet straightforward electrochemical process:
- Step 1: Water Supply & Purification High-purity, deionized water is supplied to the anode side of the PEM electrolyzer. Water purity is crucial to prevent contamination and degradation of the membrane and catalysts, ensuring long operational life.
- Step 2: Electrochemical Splitting of Water (H₂O → H₂ + O₂) at the Anode At the anode, with the application of an external DC voltage, water molecules are electrochemically oxidized:
- 2H2O → O2 + 4H+ + 4e– This reaction splits water into oxygen gas (O₂), protons (H⁺), and electrons (e⁻). The oxygen gas is channeled away.
- Step 3: Proton (H⁺) Migration Through PEM The protons (H⁺ ions) generated at the anode selectively migrate through the hydrated proton exchange membrane towards the cathode. The membrane acts as a barrier, preventing the passage of electrons and larger molecules like oxygen and hydrogen gas, thus ensuring high product purity.
- Step 4: Hydrogen Gas (H₂) Production at the Cathode At the cathode, the protons (H⁺) that have traversed the membrane combine with electrons (e⁻) supplied from the external circuit. This reduction reaction forms hydrogen gas: 4H+ + 4e– → 2H2
The high-purity hydrogen gas is then collected and channeled out of the electrolyzer. - Step 5: Oxygen Gas (O₂) Release at the Anode Simultaneously, the oxygen gas (O₂) produced at the anode is collected and vented or, in some applications, captured for other industrial uses.
The overall reaction for water electrolysis is: 2H2O → 2H2 + O2
4. Advantages of PEM Electrolyzers
PEM electrolyzers offer several distinct advantages that make them attractive for industrial applications:
- High Efficiency & Fast Response Time: PEM electrolyzers can achieve high efficiencies (typically 60-80% based on HHV of hydrogen) and possess rapid response capabilities. They can ramp up or down quickly (seconds to minutes), making them ideal for pairing with intermittent renewable energy sources like solar and wind power.
- Compact Design & Scalability: The solid electrolyte allows for a more compact cell and stack design compared to alkaline electrolyzers. This results in a smaller system footprint. PEM systems are also highly scalable, with modular designs allowing for capacity adjustments from kilowatts to multi-megawatt installations.
- High-Purity Hydrogen Output: Due to the excellent gas separation properties of the PEM, these electrolyzers produce very high-purity hydrogen (typically >99.999%) directly at the outlet, often without the need for extensive downstream purification, which is critical for applications like fuel cells or certain industrial processes. They also produce hydrogen at a higher differential pressure, reducing downstream compression costs.
- Compatibility with Renewable Energy Sources: Their dynamic operating range and fast response make PEM electrolyzers exceptionally well-suited to handle the fluctuating output of renewable energy plants, maximizing the utilization of green electricity.
- Safety and Maintenance: The use of a solid electrolyte instead of a corrosive liquid electrolyte (like KOH in alkaline systems) reduces safety concerns and simplifies maintenance requirements.
5. Industrial Applications of PEM Electrolyzers
The versatility and benefits of PEM electrolyzers have led to their adoption in a growing range of industrial applications:
- Green Hydrogen for Refineries & Ammonia Production: Replacing carbon-intensive grey hydrogen (from fossil fuels) with green hydrogen from PEM electrolyzers in refineries (for hydrocracking and desulfurization) and ammonia production (for fertilizers) is a key decarbonization pathway.
- Energy Storage for Wind & Solar Farms: PEM electrolyzers can convert surplus renewable electricity into hydrogen, which can then be stored and later converted back to electricity via fuel cells or used as a green feedstock. This helps to stabilize the grid and improve the economics of renewable energy projects.
- Fuel Cell Vehicle (FCV) Refueling Stations: High-purity hydrogen from PEM electrolyzers is essential for refueling hydrogen FCVs, including buses, trucks, and passenger cars, supporting the transition to zero-emission transportation.
- Power-to-X (PtX) Applications: Hydrogen produced by PEM electrolyzers can be a feedstock for creating synthetic fuels (e-fuels like e-methane, e-methanol, e-kerosene), green chemicals, and for direct use in industrial high-temperature heating processes.
- Industrial Feedstock: Industries such as electronics manufacturing, food processing (hydrogenation of oils), and glass production utilize high-purity hydrogen.
6. Comparison with Other Electrolyzer Technologies
Understanding how PEM electrolyzers stack up against other technologies is crucial for informed decision-making:
- PEM vs. Alkaline Electrolyzers (AEL):
- PEM Advantages: Higher current densities (more hydrogen per active area), faster response times, more compact systems, higher output pressure, higher hydrogen purity, no corrosive liquid electrolyte.
- AEL Advantages: Historically lower capital costs (though this gap is narrowing), more mature technology with a longer track record in large-scale deployments, potentially longer stack lifetimes in some conditions, less reliance on expensive platinum group metal (PGM) catalysts (though newer AELs are also incorporating advanced catalysts).
- PEM Advantages: Higher current densities (more hydrogen per active area), faster response times, more compact systems, higher output pressure, higher hydrogen purity, no corrosive liquid electrolyte.
- PEM vs. Solid Oxide Electrolyzers (SOEC):
- PEM Advantages: Lower operating temperatures (typically 50-80°C vs. >700°C for SOEC), faster startup and response, less demanding balance-of-plant materials.
- SOEC Advantages: Potentially higher electrical efficiencies, especially when integrated with high-temperature heat sources (e.g., industrial waste heat, nuclear power). Can co-electrolyze steam and CO₂ to produce syngas directly.
- SOEC Disadvantages: High operating temperatures pose material challenges and can lead to longer startup times and slower dynamic response, making them less ideal for direct coupling with highly intermittent renewables without thermal buffering.
- PEM Advantages: Lower operating temperatures (typically 50-80°C vs. >700°C for SOEC), faster startup and response, less demanding balance-of-plant materials.
7. Key Considerations for B2B Buyers
When evaluating PEM electrolyzers for industrial deployment, B2B buyers should focus on several critical factors:
- System Efficiency & Durability: Assess the overall system efficiency (not just stack efficiency) which impacts operational expenditure (OPEX). Investigate the expected lifespan of the stack and key components, as this influences the total cost of ownership and reliability. Look for proven track records and warranties.
- Maintenance Requirements: Understand the routine and unscheduled maintenance needs, including component replacement schedules (e.g., PEM stack, water filters). Lower maintenance translates to reduced downtime and operational costs.
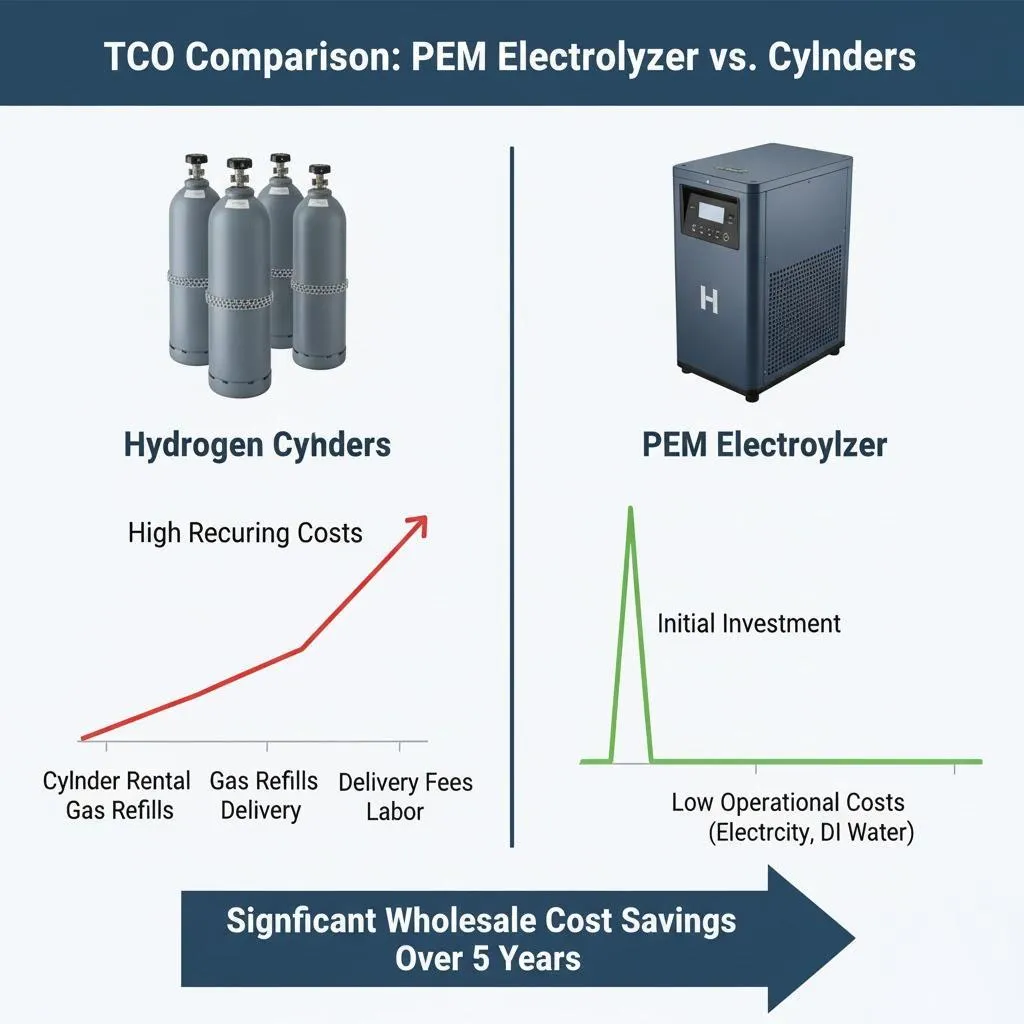
- Cost vs. ROI Analysis: Conduct a thorough techno-economic analysis. This includes:
- CAPEX (Capital Expenditure): Initial purchase price of the electrolyzer and balance-of-plant.
- OPEX (Operational Expenditure): Cost of electricity, water, maintenance, and catalyst/stack replacement over time.
- Evaluate the return on investment based on the value of the produced hydrogen (market price or avoided cost of grey hydrogen) and potential revenue from grid services.
- CAPEX (Capital Expenditure): Initial purchase price of the electrolyzer and balance-of-plant.
- Scalability and Integration: Ensure the system can be scaled to meet future demand and can be effectively integrated with existing infrastructure, renewable energy assets, and control systems.
- Supplier Credibility and Support: Choose suppliers with a strong reputation, proven technology, and comprehensive after-sales support, including installation, commissioning, and technical assistance.
8. Future Trends in PEM Electrolysis
The field of PEM electrolysis is continually evolving, with research and development focused on:
- Advances in Catalyst Materials: Significant efforts are underway to reduce or replace expensive PGM catalysts (like iridium and platinum) with more abundant and lower-cost materials without compromising performance or durability. This is key to further reducing CAPEX.
- Membrane Technology Improvements: Development of thinner, more durable, and more conductive membranes to enhance efficiency and lifespan.
- Integration with Smart Grid Systems: Enhanced control systems for better integration with smart grids, enabling PEM electrolyzers to participate in grid balancing services and optimize hydrogen production based on electricity price signals.
- Advanced Stack Design: Innovations in bipolar plate materials, GDLs, and overall stack architecture to improve performance, reduce manufacturing costs, and enhance durability.
- Direct Seawater Electrolysis: Research into PEM systems capable of directly electrolyzing seawater without extensive pre-treatment, which could open up new opportunities for offshore hydrogen production.
9. Conclusion & Call to Action
PEM electrolyzers represent a pivotal technology in the global shift towards a hydrogen-based economy. Their high efficiency, rapid response, compact design, and production of high-purity hydrogen make them exceptionally well-suited for a wide range of industrial applications, particularly when coupled with renewable energy sources for green hydrogen production.
For industrial buyers looking to invest in sustainable solutions, decarbonize operations, or tap into the growing hydrogen market, PEM electrolyzers offer a compelling and future-proof option.
Interested in PEM electrolyzers for your business? Contact our experts today for a customized solution!
[Request a Quote] or [Schedule a Consultation] with our hydrogen technology specialists.