Introduction: The Promise of Fuel Cells and the Importance of High-Purity Hydrogen
Hydrogen fuel cells represent a groundbreaking shift in the quest for sustainable and clean energy. Unlike conventional fossil fuel systems, fuel cells generate electricity through a chemical reaction between hydrogen and oxygen, emitting only water as a by-product. This makes them an attractive solution for industries striving to decarbonize transportation, electricity generation, and other energy-intensive sectors.
However, the efficiency and reliability of fuel cell systems heavily depend on the quality of the hydrogen used. High-purity hydrogen fuel cell power gas is not just a technical preference—it is a necessity. Contaminants, even in trace amounts, can degrade fuel cell performance, shorten component lifespan, and compromise safety.
This article explores why high-purity hydrogen is so vital, how it is produced, the standards it must meet, and what the future holds for hydrogen as a clean energy vector.
Fuel Cell Requirements: Why High Purity Matters
Types of Fuel Cells and Their Sensitivity to Contaminants
There are several types of fuel cells, including:
- Proton Exchange Membrane Fuel Cells (PEMFCs): Used in vehicles and portable applications. Highly sensitive to impurities.
- Solid Oxide Fuel Cells (SOFCs): Suitable for stationary power generation, slightly more tolerant of contaminants.
- Alkaline Fuel Cells (AFCs) and others: Each with unique operational characteristics and fuel purity requirements.
Common Contaminants and Their Impact
Contaminants in hydrogen can severely damage fuel cells. The most concerning include:
| Contaminant | Impact on Fuel Cell |
|---|---|
| Carbon monoxide (CO) | Poisons platinum catalyst in PEMFCs |
| Sulfur compounds | Corrode electrodes and reduce lifespan |
| Ammonia | Leads to performance degradation |
| Moisture | Disrupts membrane operation |
| Particulates | Clog flow channels and filters |
Even parts-per-million (ppm) levels of these impurities can cause irreversible damage, especially in PEMFCs. Hence, the importance of ultra-pure hydrogen cannot be overstated.
Hydrogen Purity Standards: Defining “High Purity”
International Standards for Fuel Cell Hydrogen
Organizations like the International Organization for Standardization (ISO) and SAE International have developed stringent guidelines to ensure hydrogen used in fuel cells meets quality requirements. The most widely recognized is ISO 14687, which specifies:
- CO: < 0.2 ppm
- H₂O: < 5 µmol/mol
- O₂: < 2 µmol/mol
- Total hydrocarbons: < 2 µmol/mol
Challenges in Meeting Purity Standards
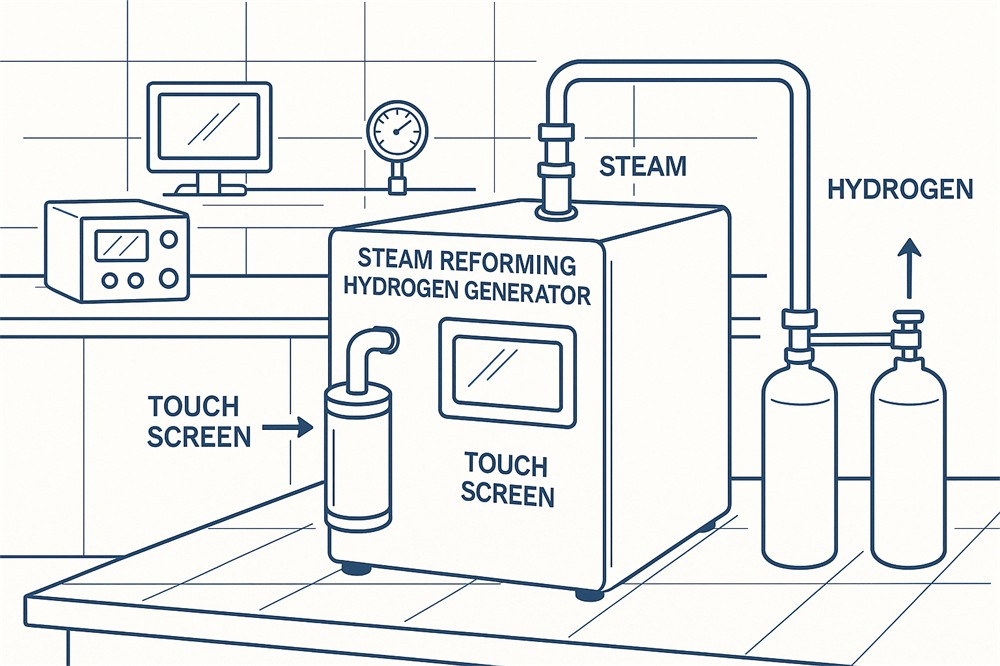
Achieving these purity levels is technically challenging and expensive. Hydrogen from common production methods like Steam Methane Reforming (SMR) contains several impurities that must be meticulously removed. Additionally, storage, transportation, and dispensing systems must maintain this purity until the hydrogen reaches the fuel cell.
Role of Real-Time Gas Analyzers
Modern systems employ on-line gas analyzers to continuously monitor hydrogen quality. These analyzers detect contaminants with extreme precision, ensuring compliance with ISO standards and preventing accidental damage to fuel cell stacks.
Production Technologies: Achieving High-Purity Hydrogen
Overview of Hydrogen Production Methods
- Steam Methane Reforming (SMR):
- Most common method globally.
- Requires extensive purification to meet fuel cell-grade purity.
- Generates CO₂ as a by-product.
- Electrolysis (Alkaline and PEM):
- Uses electricity (preferably renewable) to split water into hydrogen and oxygen.
- Cleaner than SMR but more expensive.
- Capable of producing high-purity hydrogen directly.
- Emerging Methods:
- Biomass gasification, photoelectrochemical water splitting, and others.
- Still under development but offer potential for sustainable hydrogen production.
Purification Technologies for Fuel Cell Grade Hydrogen
| Method | Key Benefits |
|---|---|
| Pressure Swing Adsorption (PSA) | Removes impurities through pressure cycling |
| Membrane Separation | Selectively allows hydrogen to pass |
| Cryogenic Purification | Cools gases to separate hydrogen |
Each method has trade-offs in terms of efficiency, cost, and scalability.
Integration with Power Systems: Enabling the Hydrogen Economy
Hydrogen and Renewable Energy Integration
When paired with solar or wind power, electrolysis enables the creation of green hydrogen—hydrogen produced without carbon emissions. This approach allows surplus renewable energy to be stored as hydrogen and later used to generate electricity when needed.
Infrastructure and Distribution Challenges
To support widespread adoption, a robust infrastructure is needed, including:
- Hydrogen fueling stations
- High-pressure pipelines
- Storage tanks and compression units
Innovations in liquid organic hydrogen carriers (LOHCs) and solid-state storage are also being explored to make hydrogen easier and safer to transport.
Real-World Applications and Case Studies
- Toyota Mirai and Hyundai Nexo use PEMFCs fueled by high-purity hydrogen.
- Hydrogen trains in Germany and fuel cell buses in California showcase scalability.
- Backup power systems in data centers demonstrate reliability and zero emissions.
Future Developments: The Road Ahead for High-Purity Hydrogen Fuel Cells
Research and Innovation
Ongoing R&D is focused on:
- Reducing electrolyzer costs
- Enhancing catalyst durability
- Scaling up green hydrogen production
Tolerant Fuel Cells: A Future Possibility?
Next-generation fuel cells may tolerate slightly lower purity levels, which could reduce hydrogen processing costs. However, this comes at the expense of reduced efficiency and higher maintenance.
Hydrogen’s Role in a Net-Zero Economy
With the global push for decarbonization, hydrogen fuel cells are poised to:
- Decarbonize heavy industries and long-haul transport
- Stabilize renewable-heavy power grids
- Provide clean off-grid electricity
Cost Forecast and Market Outlook
According to BloombergNEF, the cost of green hydrogen could drop by up to 85% by 2050, making it competitive with fossil fuels and driving broader adoption across sectors.
Frequently Asked Questions (FAQs)
1. What is high-purity hydrogen used for?
High-purity hydrogen is essential for applications like fuel cells where impurities can damage components and reduce efficiency.
2. Why are impurities in hydrogen harmful to fuel cells?
Contaminants like CO and sulfur poison the catalysts inside fuel cells, drastically reducing their lifespan and performance.
3. What is the purity level required for fuel cell hydrogen?
ISO 14687 defines the standard: <0.2 ppm CO, <2 µmol/mol O₂, and very low levels of other contaminants.
4. How is high-purity hydrogen produced?
It is produced via electrolysis or SMR followed by purification methods like PSA, membrane separation, or cryogenic distillation.
5. Can hydrogen be stored safely?
Yes, using compressed gas tanks, cryogenic liquids, or chemical storage methods such as LOHCs and metal hydrides.
6. Is green hydrogen better than blue or grey hydrogen?
Yes, green hydrogen is made using renewable electricity and has zero emissions, making it the most sustainable option.
Conclusion
The journey to a cleaner energy future is already underway, and high-purity hydrogen fuel cell power gas stands at the forefront. Ensuring hydrogen purity is not just about meeting technical specifications—it’s about unlocking the full potential of fuel cells for a decarbonized world. By investing in advanced production, purification, and distribution technologies, we can realize the vision of a sustainable hydrogen economy that powers everything from cars to cities—efficiently and emission-free.