Electrolysis is a fascinating chemical process that plays a crucial role in various industrial and scientific applications. From the extraction of metals to the production of essential chemicals, electrolysis is a cornerstone of modern chemistry. In this article, we will explore the fundamentals of electrolysis, its historical development, and its wide-ranging applications, while seamlessly integrating the target keyword, “what is electrolysis chemistry.”
What is Electrolysis in Chemistry?
Electrolysis is a chemical process that uses direct electric current (DC) to drive a non-spontaneous chemical reaction. This process is carried out in an electrolytic cell, which consists of two electrodes—an anode and a cathode—immersed in an electrolyte solution. The application of electricity causes ions in the electrolyte to move towards the oppositely charged electrodes, resulting in chemical changes. The term “electrolysis” was coined by Michael Faraday in 1834, derived from the Greek words “elektron” (amber) and “lysis” (dissolution).
The Science Behind Electrolysis
Components of Electrolysis
- Electrolyte: A substance that contains free ions and conducts electricity. It can be a liquid solution, a molten salt, or an ionic compound.
- Electrodes: Conductive materials where the oxidation and reduction reactions occur. The anode is the positive electrode where oxidation occurs, and the cathode is the negative electrode where reduction takes place.
- Power Source: A DC power supply that provides the necessary energy to drive the reaction.
Process of Electrolysis
The electrolysis process involves the transfer of electrons between the electrodes and the electrolyte. At the anode, oxidation occurs as electrons are removed from the ions or molecules, while at the cathode, reduction takes place as electrons are gained. This electron transfer results in the formation of neutral elements or new compounds.
Faraday’s Laws of Electrolysis
Faraday’s laws quantify the relationship between the amount of substance produced at an electrode and the quantity of electricity passed through the electrolyte. These laws are fundamental in calculating the efficiency and yield of electrolysis processes.
Historical Development of Electrolysis
The history of electrolysis dates back to the early 19th century, with significant contributions from scientists such as William Nicholson, Anthony Carlisle, and Humphry Davy. In 1800, Nicholson and Carlisle discovered the electrolysis of water, leading to the production of hydrogen and oxygen gases. Davy further expanded the field by isolating several elements, including potassium, sodium, and calcium, through electrolysis.
Michael Faraday’s work in the 1830s laid the foundation for the modern understanding of electrolysis, introducing key concepts and terminology still used today. The development of industrial processes, such as the Hall-Héroult process for aluminum production, further demonstrated the commercial importance of electrolysis.
Applications of Electrolysis in Industry
Electrolysis has a wide range of applications across various industries:
- Metal Extraction and Refining: Electrolysis is used to extract and purify metals from their ores. Processes like electrowinning and electrorefining are essential in producing high-purity metals such as copper, aluminum, and zinc.
- Electroplating: This process involves depositing a thin layer of metal onto a surface for protection or aesthetic purposes. Commonly used metals include gold, silver, and chromium.
- Chemical Production: Electrolysis is employed in the production of chemicals such as chlorine, hydrogen, and sodium hydroxide. The chloralkali process, for instance, produces chlorine gas and sodium hydroxide from brine.
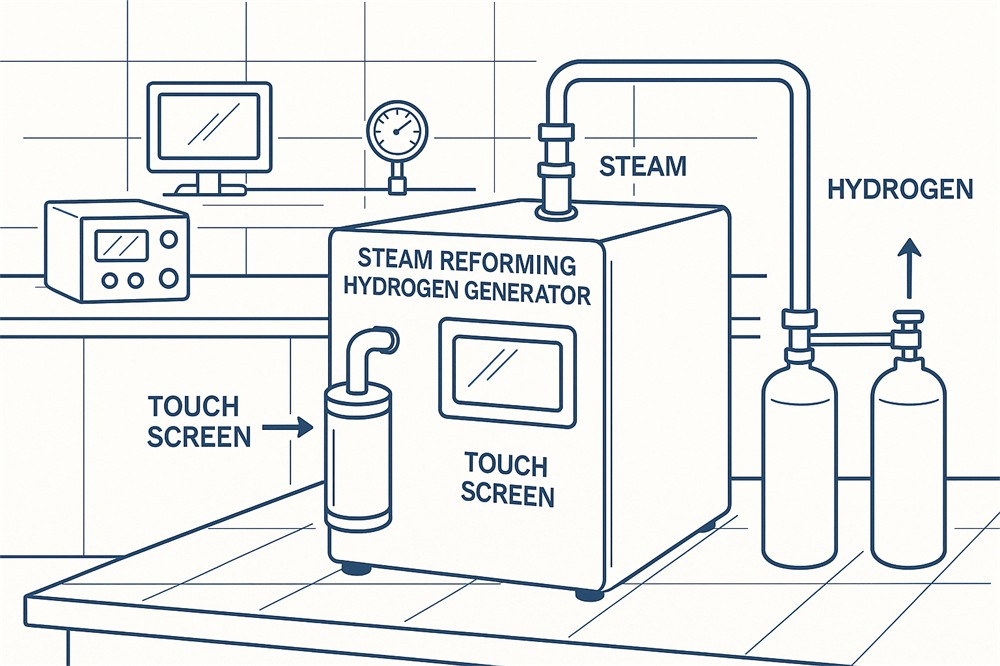
- Water Splitting: Electrolysis of water is a method to produce hydrogen and oxygen gases, which are used in fuel cells and other energy applications.
- Electrochemical Machining: This technique uses electrolysis to remove material from a workpiece, often used in precision manufacturing and metal finishing.
Safety and Environmental Considerations
While electrolysis is a powerful tool, it also poses safety and environmental challenges. Industrial-scale electrolysis requires significant energy input, contributing to carbon emissions if not sourced from renewable energy. Additionally, the process can produce hazardous byproducts, necessitating proper handling and disposal.
To mitigate these concerns, industries are exploring more sustainable practices, such as using renewable energy sources and developing processes with lower environmental impact.
Conclusion
Electrolysis is a vital process in chemistry, with applications ranging from metal extraction to chemical manufacturing. By understanding the principles and history of electrolysis, we can appreciate its impact on modern industry and science. As we continue to innovate and improve electrolysis techniques, the potential for more sustainable and efficient processes will grow, benefiting both the environment and the economy.
Whether you’re a student, researcher, or industry professional, understanding “what is electrolysis chemistry” is essential for exploring the possibilities of this transformative process. Share this article with others interested in the fascinating world of electrolysis and encourage further exploration into its applications and advancements.