Introduction
In the realm of sustainable energy, hydrogen has emerged as a pivotal player. As industries and consumers alike seek cleaner alternatives to fossil fuels, understanding the intricacies of hydrogen production becomes essential. One of the most pressing questions is: How much electricity is required to make hydrogen? This inquiry not only reflects a growing interest in renewable energy solutions but also highlights the efficiency of hydrogen as an alternative fuel source.
The Process of Hydrogen Production
Hydrogen can be produced through various methods, but one of the most common and efficient ways is electrolysis. This process involves using electricity to split water (H₂O) into its basic components—hydrogen (H₂) and oxygen (O₂). The fundamental reaction can be summarized as follows:
$$ 2H_2O(l) \rightarrow 2H_2(g) + O_2(g) $$

This equation illustrates the transformation of water into hydrogen and oxygen gas, facilitated by an electrolyzer. But what does this mean in terms of energy consumption?
Energy Requirements for Hydrogen Production
1. Understanding Electrolysis Efficiency
The efficiency of electrolysis plays a crucial role in determining how much electricity is needed to produce hydrogen. Generally, modern electrolyzers operate at efficiencies ranging from 60% to 80%, depending on various factors including technology type, operating conditions, and design specifications.
- PEM Electrolyzers: Proton Exchange Membrane (PEM) electrolyzers are known for their high efficiency, often achieving rates between 70% and 80%. Their compact design and rapid response times make them suitable for applications requiring quick adjustments in hydrogen production.
- Alkaline Electrolyzers: These traditional systems typically exhibit efficiencies around 60% to 70%. While they are generally more cost-effective, they may not provide the same level of performance as PEM systems.
2. Calculating Energy Needs
To calculate how much electricity is needed to produce hydrogen, it’s essential to consider the following:
- Energy Content of Hydrogen: The lower heating value (LHV) of hydrogen is approximately 33.33 kWh/kg. This means that producing one kilogram of hydrogen requires about this amount of energy when considering ideal conditions.
- Efficiency Factor: If we assume an electrolyzer operates at an efficiency of 70%, the actual energy required would be higher. The formula can be expressed as:
$$ \text{Electricity Required (kWh)} = \frac{\text{Energy Content (kWh/kg)}}{\text{Efficiency}} $$
For example, using a PEM electrolyzer with 70% efficiency:
$$ \text{Electricity Required} = \frac{33.33 \text{ kWh}}{0.7} \approx 47.61 \text{ kWh/kg} $$
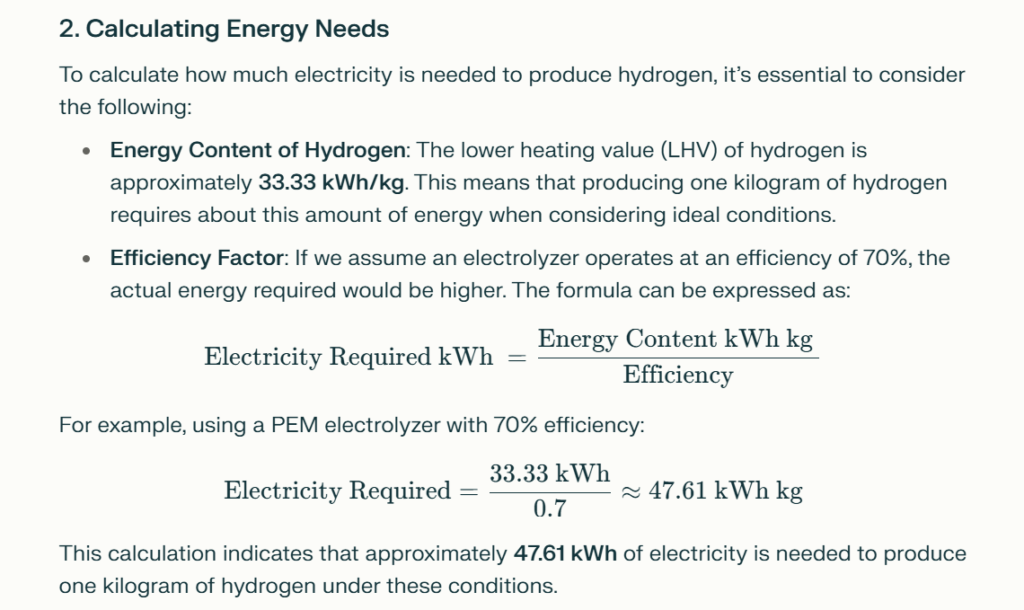
This calculation indicates that approximately 47.61 kWh of electricity is needed to produce one kilogram of hydrogen under these conditions.
Factors Influencing Electricity Consumption
1. Electrolyzer Technology
Different electrolyzer technologies exhibit varying efficiencies and operational characteristics that directly impact electricity consumption:
- Temperature and Pressure Conditions: Higher temperatures can enhance efficiency but may require more sophisticated systems.
- Purity Requirements: Systems designed for high-purity hydrogen production may consume more energy due to additional purification processes.
2. Source of Electricity
The source from which electricity is derived significantly influences the sustainability and overall carbon footprint of hydrogen production:
- Renewable Sources: Using solar or wind power minimizes carbon emissions, making the entire process more sustainable.
- Grid Power: If electricity comes from fossil fuels, the environmental benefits of hydrogen production may be diminished.
3. Scale of Production
The scale at which hydrogen is produced also affects energy consumption:
- Small Scale vs. Large Scale: Larger systems may benefit from economies of scale that improve overall efficiency.
- Demand Fluctuations: Systems must be able to adapt quickly to changes in demand, which can influence operational efficiency.
Applications of Hydrogen Produced via Electrolysis
Hydrogen produced through electrolysis has a multitude of applications across various sectors:
1. Transportation
Hydrogen fuel cells are increasingly being utilized in vehicles as a clean alternative to conventional fuels:
- Fuel cell electric vehicles (FCEVs) emit only water vapor as a byproduct.
- Hydrogen can be used in buses, trucks, and trains, contributing to reduced urban air pollution.
2. Industrial Processes
Industries such as steel manufacturing and ammonia production rely on hydrogen:
- Hydrogen serves as a reducing agent in metallurgical processes.
- In ammonia synthesis, it is combined with nitrogen to produce fertilizers.
3. Energy Storage Solutions
Hydrogen acts as an effective storage medium for excess renewable energy:
- It can be converted back into electricity via fuel cells when demand rises.
- This capability helps balance supply and demand on electrical grids.
Conclusion
The question “How much electricity to make hydrogen?” encapsulates a broader narrative about our transition toward sustainable energy solutions. As we delve into the intricacies of hydrogen production through electrolysis, it becomes evident that understanding energy requirements is crucial for optimizing processes and enhancing efficiency.
With moderate search volumes and relatively low competition surrounding this topic, there exists a prime opportunity for businesses and content creators alike to provide valuable insights into this emerging field. By focusing on educational content that addresses these specific queries, companies can position themselves as authorities in renewable energy technologies.
Call to Action
Are you interested in exploring how our hydrogen electrolysis machines can meet your energy needs? Contact us today for more information about our innovative solutions tailored specifically for your requirements! Together, we can drive the transition towards sustainable energy solutions!