Unlocking the Future of Clean Energy: A Comprehensive Guide to Hydrogen Electrolysis
As the world increasingly shifts towards sustainable energy solutions, hydrogen has emerged as a promising clean energy carrier. Among the various methods of hydrogen production, hydrogen electrolysis stands out for its potential to generate carbon-free hydrogen. This article delves into the intricacies of hydrogen electrolysis, exploring its methods, benefits, challenges, and future prospects.
What is Hydrogen Electrolysis?
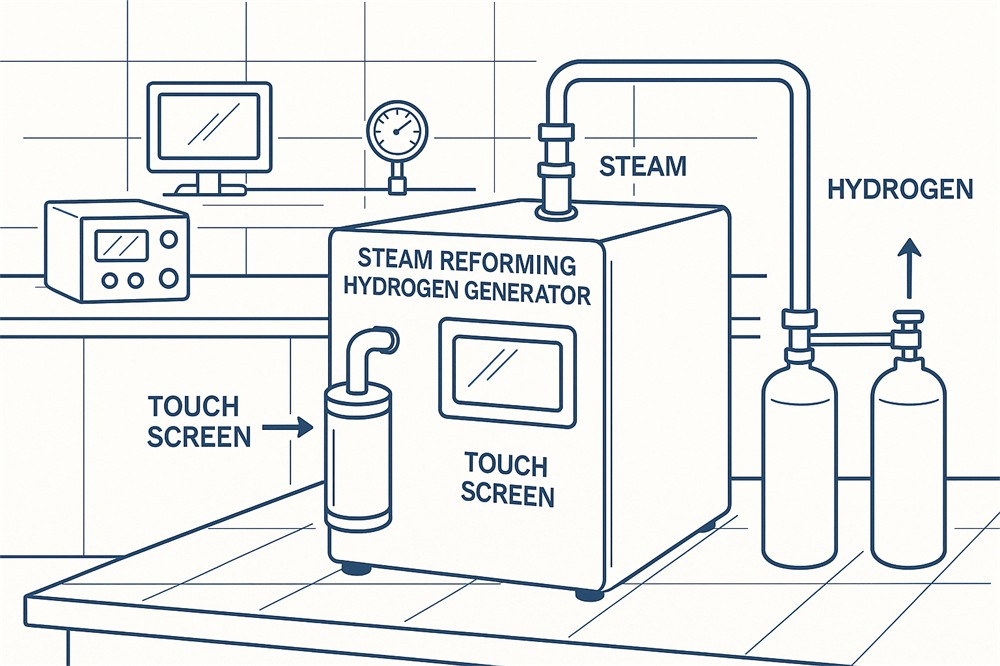
Hydrogen electrolysis is a process that uses electricity to split water (H2O) into hydrogen (H2) and oxygen (O2). This method is considered environmentally friendly because it only releases oxygen as a byproduct. The process occurs in an electrolyzer, a device composed of two electrodes—a positive electrode (anode) and a negative electrode (cathode)—submerged in water and connected to a power source.
How Does Hydrogen Electrolysis Work?
- Electric Circuit Formation: An electric current is applied to the anode and cathode, creating a circuit.
- Ion Movement: The current facilitates ion movement, with hydroxide ions (OH-) attracted to the anode and hydrogen ions (H+) to the cathode.
- Gas Generation: The anode releases oxygen, while the cathode generates hydrogen gas.
- Collection and Storage: Hydrogen gas is captured and stored, either as compressed gas or in a liquefied form.
Main Methods of Hydrogen Electrolysis
There are three primary methods of hydrogen electrolysis, each with unique characteristics:
1. Alkaline Electrolysis
Alkaline electrolysis is the most established method, using an alkaline solution, typically potassium hydroxide (KOH) or sodium hydroxide (NaOH), as the electrolyte. This method is known for its high efficiency and durability, making it suitable for large-scale industrial applications.
- Advantages: Cost-effective, uses non-precious metals, mature technology.
- Challenges: Prone to corrosion due to the aggressive alkaline solution.
2. Proton Exchange Membrane (PEM) Electrolysis
PEM electrolysis employs a solid polymer membrane as the electrolyte, offering higher efficiency and a compact design. This method is ideal for small-scale applications and dynamic renewable energy sources.
- Advantages: High efficiency, compact, dynamic response.
- Challenges: High costs due to precious metal catalysts like platinum.
3. Solid Oxide Electrolysis
Solid oxide electrolysis operates at high temperatures (above 800°C), using a ceramic-oxide electrolyte. This method is still in the research phase but holds potential for high efficiency due to the utilization of waste heat.
- Advantages: High efficiency, low operating costs with waste heat.
- Challenges: Less mature technology, requires high temperatures.
Benefits of Hydrogen Electrolysis
Hydrogen electrolysis offers several advantages over other hydrogen production methods:
- Environmental Sustainability: Produces zero greenhouse gas emissions when powered by renewable energy.
- Energy Security: Utilizes locally available resources like water and renewable electricity, reducing reliance on fossil fuels.
- Versatility: Hydrogen can be used in various applications, including fuel cells, energy storage, and industrial processes.
- Scalability: Electrolysis systems can be designed to fit different scales, from residential units to large industrial plants.
Challenges and Limitations
Despite its benefits, hydrogen electrolysis faces several challenges:
- High Capital Costs: Equipment, especially PEM and solid oxide systems, can be expensive.
- Energy Efficiency: Although improving, electrolysis is less efficient than other methods like steam methane reforming.
- Infrastructure Development: Requires robust infrastructure for hydrogen storage, transportation, and utilization.
- Competition: Must compete with established methods like steam methane reforming, which currently dominate the market.
The Role of Hydrogen Electrolysis in Green Hydrogen Production
Green hydrogen, produced using renewable energy sources, is crucial for decarbonizing various industries. Hydrogen electrolysis plays a pivotal role in this process, offering a pathway to reduce carbon emissions and enhance energy storage capabilities.
- Reduced Carbon Emissions: When powered by renewable electricity, the hydrogen produced is considered “green” and produces no direct carbon emissions.
- Energy Storage: Hydrogen can store excess electricity from renewable sources, balancing supply and demand.
- Decarbonizing Industries: Green hydrogen can replace fossil fuels in industries like steelmaking and fertilizer production.
Future Outlook
As the world embraces renewable energy, hydrogen electrolysis is expected to play a more significant role in the global energy landscape. Ongoing research aims to improve efficiency, scalability, and affordability. Supportive policies and incentives could accelerate the deployment of electrolysis systems, contributing to a cleaner, more sustainable energy future.
Conclusion
Hydrogen electrolysis presents a promising pathway for sustainable hydrogen production, with numerous applications across various sectors. While challenges remain, advancements in technology and supportive policies could lead to widespread adoption, revolutionizing industries and paving the way for a carbon-neutral future.
Call to Action
To stay updated on the latest developments in hydrogen production and electrolysis technologies, consider exploring more resources and engaging with industry experts. Share this article to spread awareness about the potential of hydrogen electrolysis in achieving a sustainable energy future.